During my holidays I visited a Hortus Botanicus in Haren (Netherlands), which is Latin for botanical garden. The park is divided into different sections having their own theme. The plants that could be found in the sections correspond with the theme, which caused the total experience to have a lot of variety.
One specific plant that grabbed my attention during the visit was the Hydrangea Macrophylla. In this name, Macrophylla means long- or large-leaved, so therefore it’s also known as bigleaf hydrangea. Yet another common name for this plant is Hortensia. The species is found in the Hydrangeaceae family and the Plantae kingdom.
What is special about the Hydrangea Macrophylla, is the variety of colors it occurs with, and this is where it becomes interesting in my opinion. Before visiting the Hortus Botanicus I already knew about the different colors and that it had something to do with the pH of the soil. In my surroundings, I mostly saw the pink-colored form, so I kind of forgot about the existence of the other colors. In the Hortus Botanicus I saw white ones and a few shades between pink and blue, all together in a specific corner specially made for this plant. This is when I realized I wanted to do some research on the different colors and how they occur.
After reading some papers and articles I discovered that what I knew, connecting color and soil pH, is indeed a correct way to explain the colors occurring. Simply said, the leaves are blue in acidic soil and pink in alkaline soil. [1] Even though this sounds cool already, it’s only the beginning!
The more detailed explanation of the color differences in the Hydrangea Macrophylla is based on the working of anthocyanins. Anthocyanins are aromatic oxygen heterocycle pigments containing a hydroxylated 2-phenylbenzopyrilium chromophore. [2,3] (A chromophore is a group in a compound that absorbs light and reflects it in a specific way so that colors occur). Cyanin means blue, so it makes sense to find anthocyanins in blue petals, but anthocyanin has a wider variety of colors than just blue. It can also exhibit colors ranging from red to purple. In general, red flowers contain different anthocyanin chromophores than blue petals. [3]
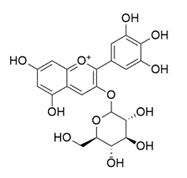
An exception is found in the Hydrangea Macrophylla. The different colors in which this plant occurs, are caused by a metalloanthocyanin. This is a pigment that is a supramolecular self-assembled metal complex that consists of the earlier described anthocyanins, flavones (a class of flavonoids; secondary metabolites), and metal ions, all in stoichiometric amounts. The name for the metalloanthocyanin in the Hydrangea Macrophylla is delphinidin-3-glucoside, which is also known as Myrtillin. The special thing about this metalloanthocyanin is that isn’t specific for red or blue like the other anthocyanins. It can exhibit shades from pink to blue, including purple. [3]
Which color the Hydrangea Macrophylla represents depends on the availability and the absorbability of Al3+ ions. These ions play a role in the metalloanthocyanin formation and therefore in the blue color of the petals. If there aren’t enough Al3+ ions, less metalloanthocyanin will be formed and then the Hydrangea Macrophylla will be pink. This is also the phenomenon on which the simple explanation of the colors, the influence of the soil pH, is based. In most soils, the amount of available aluminium is adequate, but the absorbability depends on the pH. [1] In acidic soil (pH 5.5 and lower), the Al3+ ion absorbability is good, leading to the exhibition of a blue color by the anthocyanin in its monomeric state. This results in blue-colored petals on the plant. [1,3] In neutral to alkaline soil (pH 6.5 and higher) a pink color will be present in the petals. A pH between 5.5 and 6.5 gives a combination of both, leading to purple-colored petals. Within this pH range, it’s also possible to find a combination of pink, blue and purple petals on one plant. This isn’t even unusual to find. [1]
The described metal-ion-absorbing behavior of the Hydrangea Macrophylla can take place because of the hyper tolerant hyperaccumulating character of this species. Hypertolerant describes the fact that high amounts of metals can be taken up into the roots without the plant suffering from phytotoxicity (damaged growth). Hyperaccumulating describes the translocation of the absorbed metals to organs above the ground, like leaves. Not all hyper tolerant plants are hyperaccumulating as well, but the Hydrangea Macrophylla is. [4]

So, in conclusion can be said that the Hydragea Macrophylla shows a variety of colors between pink and blue due to it’s hyperaccumulating behavior which makes supply of Al3+ ions towards the anthocyanins possible. In my opinion, a way more interesting explanation of the occurring of the colors than just pH levels in soil!
References:
[1] https://web.archive.org/ Hydrangea Questions and Answers https://web.archive.org/web/20130516083220/http://www.usna.usda.gov/Gardens/faqs/hydrangeafaq2.html#How-change-flower-color (accessed on the 28th of July 2022)
[2] Clayden, J.; Greeves, N.; Warren, S. Organic Chemistry, 2nd ed.; Oxford University Press: Oxford, 2012.
[3] Yoshida, K.; Mori, M.; Kondo, T. Blue Flower Color Development by Anthocyanins: From Chemical Structure to Cell Physiology. Natural Product Reports. July 2009, pp 884–915DOI: 10.1039/b800165k.
[4] Rascio, N.; Navari-Izzo, F. Heavy Metal Hyperaccumulating Plants: How and Why Do They Do It? And What Makes Them so Interesting? Plant Science. February 2011, pp 169–181DOI: 10.1016/j.plantsci.2010.08.016.






















Comentarios